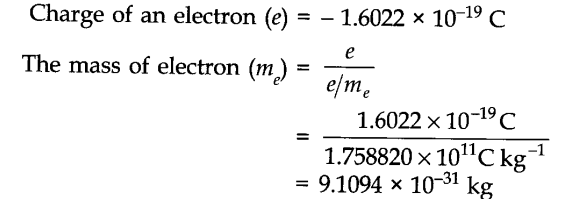
31) The mass of an electron is approximately 1/1836 times the mass of A) 1/1H B) 2/1H c) 3/1H D) - Brainly.com
The mass of half mole of electrons is about : (B) 0.273 mg3.(C) 1.092 mg(D) 4.55 mg(A) 0.546 mg39.4 kg

GRE Physics - ✊#Calculation_Using_Concept_E=mc^2 ✋#Question Calculate the kinetic energy and total energy of electron moving with velocity 0.98 times the velocity of light in the laboratory system. #Note_To_Understand. ✌Relativistic mass: Mass increased
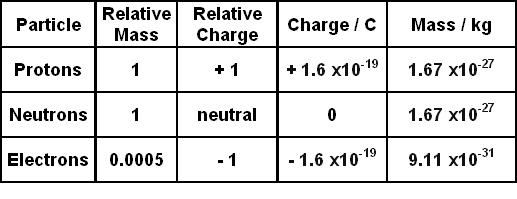
The mass of an electron can be expressed as (A) 0.512 MeV (B) 8.19 × 10–14J/c2 (C) 9.1 × 10–31 kg (D) 0.00055 amu
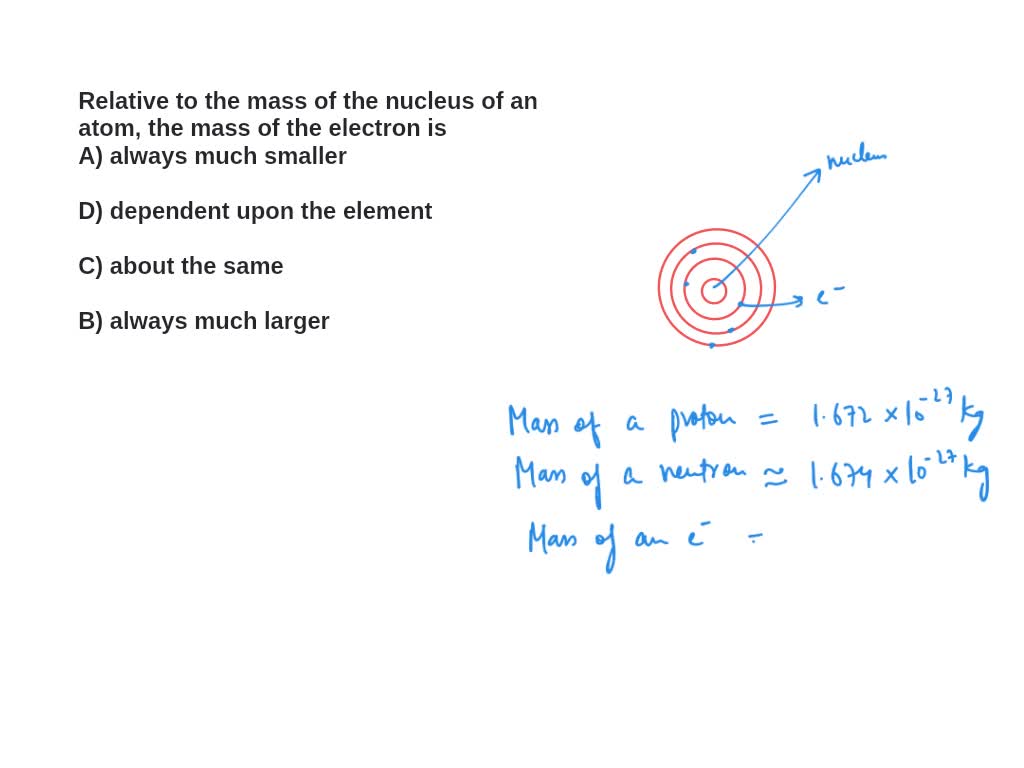








.PNG)